Main page
About us
Sliding Bearings Consulting
Advertising Opportunities

to Metals
to Basic processes in metals
Solidification
Dr. Dmitri Kopeliovich
Solidification is a comprehensive process of transformation of the melt of an alloy into a solid piece of the alloy, involving crystallization of the liquid phase, segregation of impurities and alloying elements, liberation of the gases dissolved in the melt, shrinkage cavities and porosity formation.
Structure of ingots and castings
Fine and homogeneous grain structure is the most desirable for the common castings and ingots.
It is achieved when the crystallization proceeds under the following conditions:
- Formation of a large number of stable nuclei;
- Fast extraction of latent crystallization heat and the superheat of the liquid.
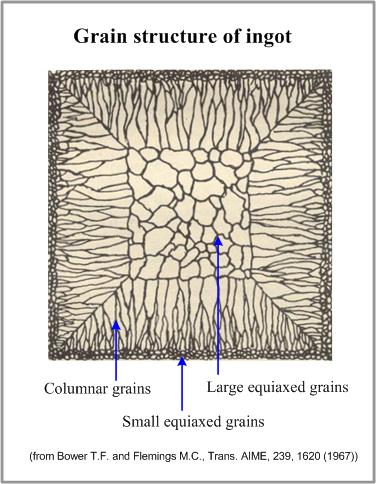
These conditions are realized when a melt comes to a contact with a wall of a cold metallic mold. Small equiaxed grains (chill crystals) form at this stage. Latent crystallization heat, liberating from the crystallizing metal, decreases the undercooling of the melt and depresses the fast grains growth.
At this stage some of small grains, having favorable growth axis, start to grow in the direction opposite to the direction of heat flow. As a result columnar crystals (columnar grains) form. Length of the columnar grains zone is determined by the constitutional undercooling. When the temperature of the melt, adjacent to the solidification front, increases due to the liberation of the latent heat, constitutional undercooling will end and the columnar grains growth will stop.
Further cooling of the molten alloy in the central zone of the ingot will cause formation of large equiaxed grains.
Formation of the grain zones of an ingot is presented in the figure.
The crystals, growing as a result of solidification of ordinary alloys, are in dendrite form.
to top
Segregation
Composition of solidified alloy is not uniform. Concentrations of impurities and alloying elements are different in different parts of the casting. This difference is a result of different solubility of impurities in liquid and solid phases at the equilibrium temperature.
Segregation is a result of separation of impurities and alloying elements in different casting regions.
Microsegregation is a segregation of impurities between the dendrite arms. This kind of segregation may be considerably diminished by diffusion of the impurities atom into the dendrite arms during homogenizing annealing.
Advancing the solidification front towards the ingot center causes enrichment of the liquid in the central zone by impurities and alloying additives, rejected by the solidifying metal and pushed by the solidification front. Segregated impurities are arranged as V-shape marks on the vertical section of the ingot. This effect is called normal macrosegregation.
Gravity segregation is a segregation caused by precipitation of primary crystals, which are heavier, than the melt.
to top
Gas pores
Gas pores, entrapped in the solid structure of a casting, arise from different origins:
- Gas (Hydrogen) dissolved in the liquid during melting (from damp materials, atmosphere, oils, etc). When the melt cools down and solidifies hydrogen solubility decreases and it is forced out from the melt. The gas bubbles are trapped by the dendrites, forming gas porosity.
- Gas pores, called blowholes, may be a result of chemical reaction occurring in the solidifying alloy. If a liquid steel was not deoxidized by deoxidizers (aluminum, silicon), Oxygen and carbon, which are solved in the steel, form carbon monoxide by the reaction: C + O = CO. The bubbles of CO, trapped by the dendrites, form blowholes.
- Surface blowholes may form as a result of the decomposition of some constituents of mold dressing.
Shrinkage
Shrinkage is a contraction of alloy volume caused by:
- Contraction of the melt when it cools down to the liquidus temperature;
- Contraction of the alloy owing its solidification (cooling from liquidus temperature to solidus temperature). All metals except bismuth have higher density in solid state, than in liquid.
- Contraction of the solid alloy cooling from the solidus temperature to the ambient temperature.
Shrinkage cavity
When a large isolated region of liquid phase remains within solid, surrounding it, shrinkage cavity will form in this region. The common mold structure includes a riser – a “head”, in which the melt solidifies last and “feeds” the main casting with liquid alloy, compensating the casting shrinkage.
Shrinkage porosity
This shrinkage defect is a characteristic for the central regions of castings (ingots) of the alloys with a wide temperature range of solidification. In these castings “feeding” melt is not able to infiltrate through the interlacing dendrites. The local micro-spaces between the dendrites arm remain isolated from the melt in riser forming micro-cavities or shrinkage porosity.
to top
Related internal links