Main page
About us
Sliding Bearings Consulting
Advertising Opportunities

to Metals
to Fundamentals of metals
Solid solutions
Alloy
Alloy is a metal, composing of a mixture of elements. Most of alloys are composed of a base metal with small amounts of additives or alloying elements. The typical examples of alloys are steel/cast iron (iron base alloys), bronze/brass (copper base alloys), aluminum alloys, nickel base alloys, magnesium base alloys, titanium alloys.
Alloys may be prepared by different technological methods: melting, sintering of a powders mixture, high temperature diffusion of an alloying element into the base metal, plasma and vapor deposition of different elements, electroplating etc.
Alloy structure may be a single phase or a multi phase.
to top
Phase
Phase is a uniform part of an alloy, having a certain chemical composition and structure, and which is separated from other alloy constituents by a phase boundary.
An alloy phase may be in form of valence compound (substance formed from two or more elements), with a fixed ratio determining the composition) or in form of solid solution.
Solid solution is a phase, where two or more elements are completely soluble in each other.
Depending on the ratio of the solvent (matrix) metal atom size and solute element atom size, two types of solid solutions may be formed: substitution or interstitial.
to top
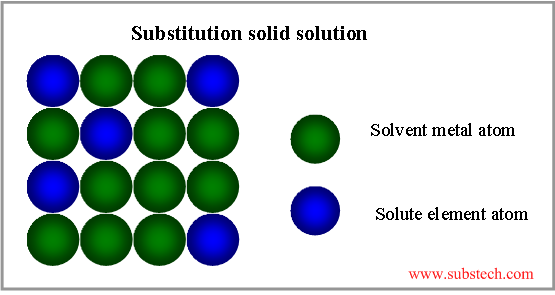
Substitution solid solution
If the atoms of the solvent metal and solute element are of similar sizes (not more, than 15% difference), they form substitution solid solution, where part of the solvent atoms are substituted by atoms of the alloying element (see the picture below).
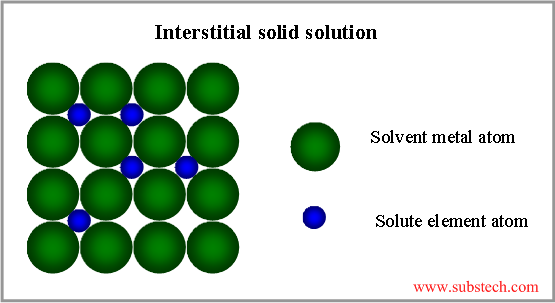
Interstitial solid solution
If the atoms of the alloying elements are considerably smaller, than the atoms of the matrix metal, interstitial solid solution forms, where the matrix solute atoms are located in the spaces between large solvent atoms (see the picture below).
When the solubility of a solute element in interstitial solution is exceeded, a phase of intermediate compound forms. These compounds (TiN, WC, Fe3C etc.) play important role in strengthening steels and other alloys.
Some substitution solid solutions may form ordered phase where ratio between concentration of matrix atoms and concentration of alloying atoms is close to simple numbers like AuCu3 and AuCu.
Solid solution formation usually causes increase of electrical resistance and mechanical strength and decrease of plasticity of the alloy.
to top
Related internal links