Main page
About us
Sliding Bearings Consulting
Advertising Opportunities

to Metals
to Basic processes in metals
Basic principles of heat treatment
Dr. Dmitri Kopeliovich
Heat treatment of a metal or alloy is a technological procedure, including controlled heating and cooling operations, conducted for the purpose of changing the alloy microstructure and resulting in achieving required properties.
There are two general objectives of heat treatment:
Hardening
Hardening is a process of increasing the metal hardness, strength, toughness, fatigue resistance.
- Strain hardening (work hardening) – strengthening by cold-work (cold plastic deformation).
Cold plastic deformation causes increase of concentration of dislocations, which mutually entangle one another, making further dislocation motion difficult and therefore resisting the deformation or increasing the metal strength.
- Grain size strengthening (hardening) – strengthening by grain refining.
Grain boundaries serve as barriers to dislocations, raising the stress required to cause plastic deformation.
- Solid solution hardening – strengthening by dissolving an alloying element.
Atoms of solute element distort the crystal lattice, resisting the dislocations motion. Interstitial elements are more effective in solid solution hardening, than substitution elements.
- Dispersion strengthening – strengthening by addition of second phase into metal matrix.
The second phase boundaries resist the dislocations motions, increasing the material strength. The strengthening effect may be significant if fine hard particles are added to a soft ductile matrix (composite materials).
- Hardening as a result of Spinodal decomposition. Spinodal structure is characterized by strains on the coherent boundaries between the spinodal phases causing hardening of the alloy.
- Precipitation hardening (age hardening) – strengthening by precipitation of fine particles of a second phase from a supersaturated solid solution.
The second phase boundaries resist the dislocations motions, increasing the material strength.
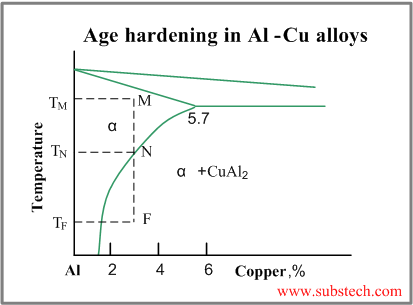
The age hardening mechanism in Al-Cu alloys may be illustrated by the phase diagram of Al-Cu system (see figure below)
When an alloy Al-3%Cu is heated up to the temperature TM, all CuAl2 particles are dissolved and the alloy exists in form of single phase solid solution (α-phase). This operation is called solution treatment.
Slow cooling of the alloy will cause formation of relatively coarse particles of CuAl2 intermetallic phase, starting from the temperature TN.
However if the the cooling rate is high (quenching), solid solution will retain even at room temperature TF. Solid solution in this non-equilibrium state is called supersaturated solid solution.
Obtaining of supersaturated solid solution is possible when cooling is considerably faster, than diffusion processes.
As the diffusion coefficient is strongly dependent on the temperature, the precipitation of CuAl2 from supersaturated solution is much faster at elevated temperatures (lower than TN).This process is called artificial aging. It takes usually a time from several hours to one day.
When the aging is conducted at the room temperature, it is called natural aging. Natural aging takes several days or more.
Precipitation from supersaturated solid solution occurred in several steps:
- Segregation of Cu atoms into plane clusters. These clusters are called Guinier-Preston1 zones (G-P1 zones).
- Diffusion of Cu atoms to the G-P1 zones and formation larger clusters, called GP2 zones or θ” phase. This phase is coherent with the matrix .
- Formation of θ’ phase which is partially coherent with the matrix. This phase provides maximum hardening.
Annealing
Annealing is a heat treatment procedure involving heating the alloy and holding it at a certain temperature (annealing temperature), followed by controlled cooling.
Annealing results in relief of internal stresses, softening, chemical homogenizing and transformation of the grain structure into more stable state.
Annealing stages:
- Stress relief (recovery) – a relatively low temperature process of reducing internal mechanical stresses, caused by cold-work, casting or welding.
During this process atoms move to more stable positions in the crystal lattice. Vacancies and interstitial defects are eliminated and some dislocations are annihilated.
Recovery heat treatment is used mainly for preventing stress-corrosion cracking and decreasing distortions, caused by internal stresses.
- Recrystallization – alteration of the grain structure of the metal.
If the alloy reaches a particular temperature (recrystallization or annealing temperature) new grains start to grow from the nuclei formed in the cold worked metal. The new grains absorb imperfections and distortions caused by cold deformation. The grains are equi-axed and independent to the old grain structure.
As a result of recrystallization mechanical properties (strength, ductility) of the alloy return to the pre-cold-work level.
The annealing temperature and the new grains size are dependent on the degree of cold-work which has been conducted. The more the cold-work degree, the lower the annealing temperature and the fine recrystallization grain structure. Low degrees of cold-work (less than 5%) may cause formation of large grains.
Usually the annealing temperature of metals is between one-third to one-half of the freezing point measured in Kelvin (absolute) temperature scale.
- Grain growth (over-annealing, secondary recrystallization) – growth of the new grains at the expense of their neighbors, occurring at temperature, above the recrystallization temperature.
This process results in coarsening grain structure and is undesirable.
to top
Related internal links