Main page
About us
Sliding Bearings Consulting
Advertising Opportunities

to Metals
to Steel making
Desulfurization of steel
Sulfur in steel
Sulfur (S) may dissolve in liquid iron (Fe) at any concentration. However solubility of sulfur in solid iron is limited: 0.002% in α-iron at room temperature and 0.013% in γ-iron at 1832°F (1000°C).
When a liquid steel cools down and solidifies the solubility of sulfur drops and it is liberated from the solution in form of iron sulfide (FeS) forming an eutectic with the surrounding iron. The eutectic is segregated at the iron grain boundaries. The eutectic temperature is relatively low - about 1810°F (988°C).
Fe-FeS eutectic weakens the bonding between the grains and causes sharp drop of the steel properties (brittleness) at the temperatures of hot deformation (Rolling, Forging etc.).
Brittleness of steel at hot metal forming operations due to the presence of low-melting iron sulfides segregated at grain boundaries is called hot shortness.
In order to prevent formation of low-melting iron sulfide manganese (Mn) is added to steel to a content not less than 0.2%.
Manganese actively reacts with iron sulfides during solidification of steel transforming FeS to MnS according to the reaction:
(FeS) + [Mn] = (MnS) + Fe
(square brackets [ ] - signify concentration in steel, round brackets ( ) signify concentration in slag)
The melting temperature of manganese sulfide is relatively high - about 2930°F (1610°C) therefore the steels containing manganese may be deformed in hot state (no hot shortness).
Unfortunately MnS inclusions are:
- Brittle (less ductile than steel);
- They may have sharp edges;
- They are located between the steel grains.
All these factors determine negative influence of sulfide inclusions on the mechanical properties. Cracks may be initiated at brittle sharp edge inclusions. Sulfide inclusions especially arranged in a chain form also make easier the cracks propagation along the grain boundaries.
The negative effect of sulfur on the steel properties becomes more significant in large ingots and castings, some zones of which are enriched by sulfur (macrosegregation of sulfur).
The properties negatively affected by sulfur:
- corrosion resistance;
- Weldability.
Desulfurization of steel by slags
The most popular method of desulfurization is removal of sulfur from molten steel to the basic reducing slag. Basic slag is a slag containing mainly basic oxides: CaO, MgO, MnO, FeO.
A typical basic slag consists of 35-60% CaO + MgO, 10-25% FeO, 15-30% SiO2, 5-20% MnO.
Transition of sulfur from steel to slag may be presented by the chemical equation:
[S] + (CaO) = (CaS) + [O]
The equilibrium constant KS1 of the reaction is:
KS1 = a[O]*a(CaS)/a[S]*a(CaO)
Where:
a[O], a[S] - activities of oxygen and sulfur in the liquid steel;
a(CaS), a(CaO) - activities of CaS and CaO in the slag.
The same reaction in ionic form:
[S] + (O2-) = (S2-) + [O]
The equilibrium constant KS2 of the reaction is:
KS2 = a[O]*a(S2-)/a[S]*a(O2-)
Where:
a(S2-), a(O2-) - activities of S2- and O2- in the slag.
Capability of a slag to remove sulfur from steel is characterized by the distribution coefficient of sulfur:
LS = (S)/[S]
Where:
(S) - concentration of sulfur in slag;
[S] - concentration of sulfur in steel;
As appears from the above equations desulfurization is effective in deoxidized (low (O)) basic (high (CaO)) slags. Therefore ability of Basic Oxygen Process (BOP) to remove sulfur is low due to its highly oxidized slag.
Desulfurization may be effectively conducted in the reducing slag stage of the steel making process in Electric-arc furnace. At this stage the oxidizing slag is removed and then lime flux is added to form basic slag with high CaO content.
Deep desulfurization by slags may be achieved in ladle:
- The refining (desulfurizing) slag with high content of CaO and no FeO is prepared and placed in an empty ladle.
- The molten steel is poured into the ladle filled with the refining slag.
- Energy of the falling steel stream causes mixing the slag with the steel, during which sulfur is removed from the steel to slag phase.
Effect of desulfurization may be enhanced by additional stirring, for which electromagnetic (induction) stirrers or argon bubbling are used.
to top
Desulfurization of steel by injection of active agents
Injection of desulfurizing agents to a molten steel is the most effective method of sulfur removal.
Injection methods usually combine supply of a disperse desulfurizing agent (powder) with stirring by argon blowing.
Deep desulfurization by injection of active agents are achieved due to the following factors:
- High chemical activity of the desulfurization agents (Ca, Mg);
- High contact area between the steel and slag phases;
- Stirring providing good kinetic conditions of desulfurization;
- Presence of basic non-oxidized slag capable to absorb the products of the desulfurization reaction (CaS, MgS).
The following materials are used as desulfurizing agents:
- Slag mixtures CaO (50-90%) + CaF2 (10-20%) + A2lO3 (0-30%);
- CaSi;
- CaC2;
- CaC2 + Mg;
- Lime (CaO) + Mg;
- Ca + Al;
- Ca;
- Mg.
The desulfurizing agents are injected into molten steel either in form of powder transported by an argon blown to the steel through a lance or in form of a cored wire containing powder of desulfurizing agent. In the latter method stirring by argon bubbling from the porous plug mounted in the ladle bottom is used.
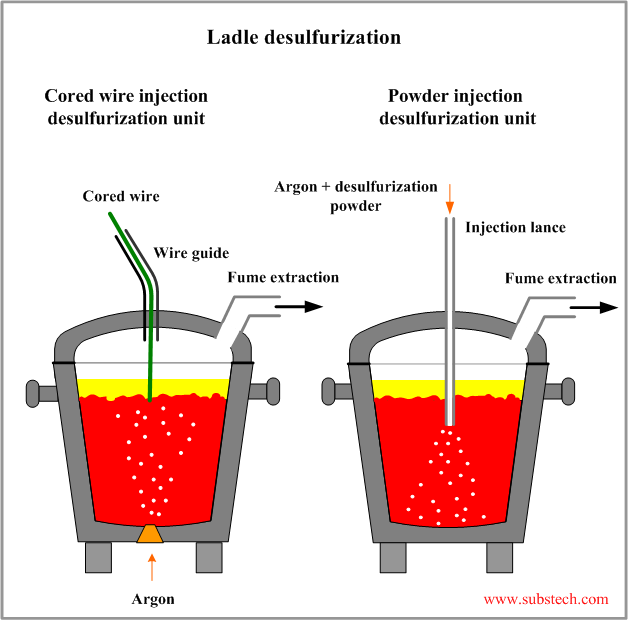
Chemical reactions between desulfurizing agents and sulfur dissolved in steel may be presented by the following equations:
Ca + [S] = (CaS)
Mg + [S] = (MgS)
Injection of desulfurizing agents allows to achieve ultra-low concentrations of sulfur in steel (0.0002%).
to top
Related internal links
to Metals
to Steel making


