Main page
About us
Sliding Bearings Consulting
Advertising Opportunities

to Fluids
to Dispersions
Surfactants
Dr. Dmitri Kopeliovich
Surfactant (surface active agent,detergent) is a substance reducing the interfacial energy of the solution in a contact with other phases.
Surfactants promote dispersion of various phases: emulsification of oils (liquid phase), suspension of solid residuals (solid phase) in the alkaline cleaners and foaming (gaseous phase).
Amphiphilic structure of surfactant molecules
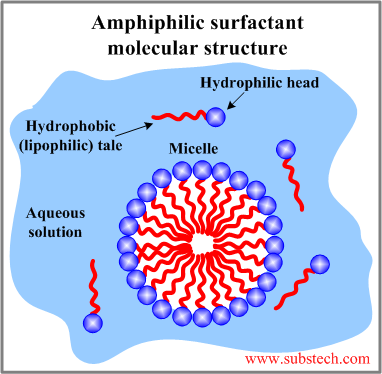 The ability of surfactants to reduce the interfacial energy is related to the polar-nonpolar structure of their molecules. A surfactant molecule consists of two different groups: a hydrophilic head and a hydrophobic tail. Such dual structure of surfactants is essential for reduction of the energy on the interface between a polar and non-polar phases.
The ability of surfactants to reduce the interfacial energy is related to the polar-nonpolar structure of their molecules. A surfactant molecule consists of two different groups: a hydrophilic head and a hydrophobic tail. Such dual structure of surfactants is essential for reduction of the energy on the interface between a polar and non-polar phases.
The hydrophilic (water-loving) heads of surfactant molecules are polar therefore they are attracted by the molecules of polar Solvents. A typical polar solvent is water. A water molecule is a dipole - it has a positively charged portion (Hydrogen atoms) and a negatively charged portion (Oxygen atom). Other polar substances are readily dissolved in water due to their strong affinity to the polar water molecules. The polar heads are repelled by the non-polar molecules such as oil molecules therefore hydrophilic portions are also lipophobic (fat-hating).
The hydrophobic (water-hating) tails of surfactant molecules are non-polar therefore they are repelled by polar water molecules but they have an affinity to non-polar molecules of oils and fats. The hydrophobic tails are lipophilic (fat-loving).
The affinity of surfactant molecules is dual or amphiphilic (double-loving).
Due to their amphiphilic nature surfactant molecules are repealed to the interface in any solvent (either polar or non-polar).
In a mixture of a polar solvent (e.g., aqueous solution) with a non-polar substance (e.g., oil) the surfactant molecules are arranged on the interface with the polar heads in the solvent whereas the non-polar tails are oriented out of the solvent in the oil phase.
Surfactant molecules in an aqueous solution may form a spherical aggregates called micelles. In a micelle the surfactant molecules are radially oriented with the hydrophilic groups at the micelle surface and with the end of the hydrophobic tails in the center.
The micelle formation (micellization) is possible if the surfactant concentration is above the Critical Micelle Concentration (CMC).
to top
Anionic surfactants
Anionic surfactants dissociate in aqueous solutions into a amphiphilic organic anion (negatively charged ion) and a small inorganic cation (positively charged ion: Na+, K+).
Anionic surfacants are manufactured in the reaction of an organic substance with sodium/potassium hydroxide.
Examples of anionic surfactants: linear alkylbenzene sulfonates, di-alkyl sulfosuccinate (sulfonic acid salt), sodium lauryl sulfate (alcohol sulfate), phosphoric acid esters, sodium stearate (carboxylic acid salt).
Soaps (salts of fatty acids) are typical anionic surfactants.
Anionic surfacants are generally non-toxic.
Anionic surfactants are most widely used surfactants.
to top
Cationic surfactants
Cationic surfactants dissociate in aqueous solutions into an amphiphilic cation (positively charged ion) and an halide anion (negatively charged halogen ion).
Cationic surfactants are manufactured in the reaction between alkyl halides with fatty amines.
They are are widely used for treatment of synthetic fabrics and proteins molecules of which have negatively charged sites.
Cationic surfactants are effective in acidic solutions and are not effective in alkaline solutions.
As compared to the anionic surfactants the cationic ones are more expensive and more toxic.
to top
Non-ionic surfactants
Non-ionic surfactants do not dissociate (do not form anions and cations) in aqueous solutions.
The hydrophilic groups of non-ionic surfactants are produced by ethoxylation - an addition of ethylene oxide to alcohol, phenol, amides, ether or ester.
The hydrophobic group are alkyl or alkylbenzene type.
The examples of non-ionic surfactants: alkylphenol ethoxylates, alcohol ethoxylates, alkanolamides.
Non-ionic surfactants are insensitive to the water PH and hardness. They are the second widely used after anionic surfactants.
to top
Amphoteric surfactants
Amphoteric surfactants may be either anionic, cationic or no-ionic depending on the PH level of the aqueous solution.
A molecule of an amphoteric surfactant consists of a hydrophobic (lipophilic) tail and a hydrophilic portion having a properties of a zwitterion (a molecule with positive and a negative electrical charge at different locations).
Amphoteric surfactants are bio-compatible and non-toxic however their use is limited by the relatively high cost.
Examples of amphoteric surfactants: sodium lauriminodipropionate and disodium lauroamphodiacetate.
to top
Related internal links


