Main page
About us
Sliding Bearings Consulting
Advertising Opportunities

to Fluids
to Lubricants
Boron nitride as solid lubricant
Dr. Dmitri Kopeliovich
Boron nitride may exist in two forms of crystal lattice: cubic and hexagonal.
Due to its tight diamond-like structure cubic boron nitride is extremely hard. It has poor lubrication properties and is used in cutting and abrasive tools as a diamond substitute.
Hexagonal boron nitride (HBN) is a solid lubricant relating to the class of Inorganic lubricants with lamellar structure, which also includes molybdenum disulphide, graphite and some other sulphides, selenides and tellurides (chalcogenides) of molybdenum, tungsten, niobium, tantalum and titanium.
The crystal lattice of hexagonal boron nitride consists of hexagonal rings forming thin parallel planes. Atoms of boron (B) and nitrogen (N) are covalently bonded to other atoms in the plane with the angle 120 ° between two bonds (each boron atom is bonded to three nitrogen atoms and each nitrogen atom is bonded to three boron atoms).
The planes are bonded to each other by weak Van der Waals forces.
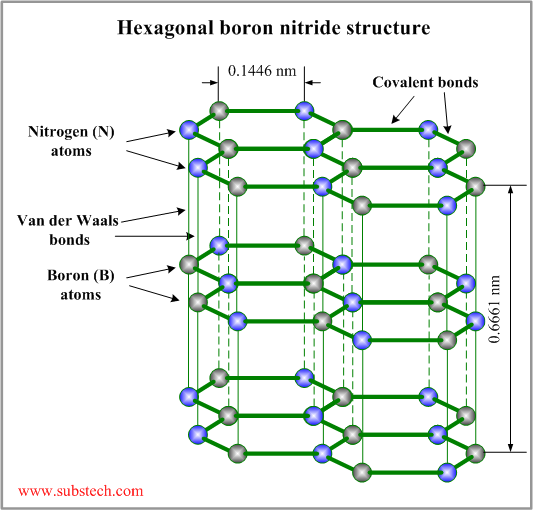
The layered structure allows sliding movement of the parallel planes. Weak bonding between the planes provides low shear strength in the direction of the sliding movement but high compression strength in the direction perpendicular to the sliding movement.
Friction forces cause the particles of boron nitride to orient in the direction, in which the planes are parallel to the sliding movement. The anisotropy of the mechanical properties imparts the combination of low coefficient of friction and high carrying load capacity to boron nitride.
Boron nitride forms a lubrication film strongly adhered to the substrate surface. The lubrication film provides good wear resistance and seizure resistance (compatibility).
Similar to molibdenum disulfide moist atmosphere is not required for lubrication by boron nitride. It demonstrates low friction in dry atmosphere and in vacuum.
Coefficient of friction of boron nitride is within the range 0.1-0.7, which is similar to that of graphite and molybdenum disulfide. Impurities (eg. boron oxide) exert adverse effect on the lubrication properties of boron nitride.
Boron nitride is chemically inert substance. It is non-reactive to most acids, alkalis, solvents and non-wetted by molten aluminum, magnesium, molten salts and glass.
The main advantage of boron nitride as compared to graphite and molybdenum disulfide is its thermal stability. Hexagonal boron nitride retains its lubrication properties up to 5000°F (2760°C) in inert or reducing environment and up to 1600°F (870°C) in oxidizing atmosphere.
Boron nitride has high thermal conductivity.
Some applications of hexagonal boron nitride:
- Components of polymer based composite anti-friction coatings
- Second phase particles of metal based composite anti-friction coatings
- Solid lubricant in metal forming
- Release coatings and non-sticking refractory linings in foundry
- Sintered ceramic parts for high temperature applications
Related internal links
to Fluids
to Lubricants


