to Metals
to Steel making
Steel making (introduction)
Dr. Dmitri Kopeliovich
Steel is an alloy of iron, containing up to 2% of carbon (usually up to 1%).
Steel contains lower (compared to pig iron) quantities of impurities like phosphorous, sulfur and silicon.
Steel is produced from pig iron by processes, involving reducing the amounts of carbon, silicon and phosphorous.
The main steel making methods are:
Basic Oxygen Process (BOP)
The Basic Oxygen Process is the most powerful and effective method of steel manufacturing.
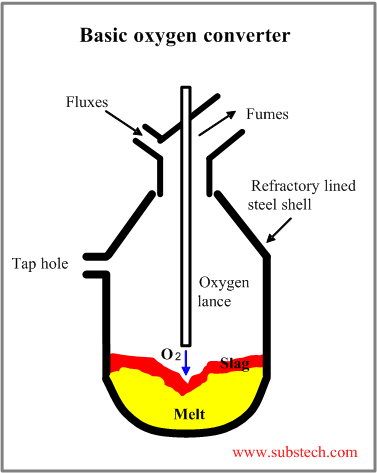
The scheme of the Basic Oxygen Furnace (BOF) (basic oxygen furnace, basic oxygen converter) is presented in the picture.
Typical basic oxygen converter has a vertical steel shell lined with refractory lining.
The furnace is capable to rotate about its horizontal axis on trunnions.
This rotation is necessary for charging raw materials and fluxes, sampling the melt and pouring the steel and the slag out of the furnace.
The Basic Oxygen is equipped with the water cooled oxygen lance for blowing oxygen into the melt.
The basic oxygen converter uses no additional fuel. The pig iron impurities (carbon, silicon, manganese and phosphorous) serve as fuel.
The steel making process in the oxygen converter consists of:
- Charging steel scrap.
- Pouring liquid pig iron into the furnace.
- Charging fluxes.
- Oxygen blowing.
- Sampling and temperature measurement
- Tapping the steel to a ladle.
- De-slagging.
The iron impurities oxidize, evolving heat, necessary for the process.
The forming oxides and sulfur are absorbed by the slag.
The oxygen converter has a capacity up to 400 t and production cycle of about 40 min.
to top
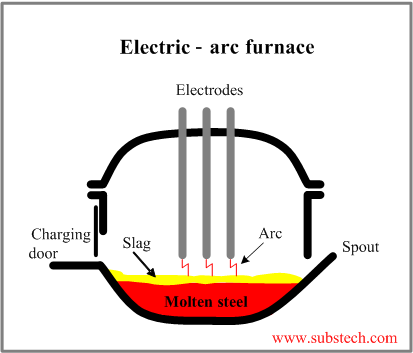
Electric-arc furnace
The electric-arc furnace employs three vertical graphite electrodes for producing arcs, striking on to the charge and heating it to the required temperature.
As the electric-arc furnace utilizes the external origin of energy (electric current), it is capable to melt up to 100% of steel scrap.
The steel making process in the electric-arc furnace consists of:
- Charging scrap metal, pig iron, limestone
- Lowering the electrodes and starting the power (melting)
- Oxidizing stage
At this stage the heat, produced by the arcs, causes oxidizing phosphorous, silicon and manganese. The oxides are absorbed into the slag. By the end of the stage the slag is removed.
- De-slagging
- Reducing stage
New fluxes (lime and anthracite) are added at this stage for formation of basic reducing slag.
The function of this slag is refining of the steel from sulfur and absorption of oxides, formed as a result of deoxidation.
- Tapping
- Lining maintenance
The advantages of the electric-arc furnace are as follows:
- Unlimited scrap quantity may be melt;
- Easy temperature control;
- Deep desulfurization;
- Precise alloying.
Ladle refining (ladle metallurgy, secondary refining)
Ladle refining is post steel making technological operations, performed in the ladle prior to casting with the purposes of desulfurization, degassing, temperature and chemical homogenization, deoxidation and others.
Ladle refining may be carried out at atmospheric pressure, at vacuum, may involve heating, gas purging and stirring.
Sulfur refining (desulfurization) in the ladle metallurgy is performed by addition of fluxes (CaO, CaF2 and others) into the ladle and stirring the steel together with the slag, absorbing sulfur.
In the production of high quality steel the operation of vacuum treatment in ladle is widely used.
Vacuum causes proceeding chemical reaction within the molten steel:
[C] + [O] = {CO}
This reaction results in reduction of the quantity of oxide inclusions.
The bubbles of carbon oxide remove Hydrogen, diffusing into the CO phase.
An example of ladle refining method is Recirculation Degassing (RH) vacuum degasser, which consists of a vacuum vessel with two tubes (snorkels), immersed in the steel.
In one of the tubes argon is injected.
Argon bubbles, moving upwards, cause steel circulation through the vacuum vessel. Additions of fluxes in the vacuum vessel permits conducting desulfurization treatment by this method.
to top
Related internal links
to Metals
to Steel making