Main page
About us
Sliding Bearings Consulting
Advertising Opportunities

to Ceramics
to Carbon materials
Synthetic diamonds
Dr. Dmitri Kopeliovich
Diamond is a crystalline, transparent and extremely hard allotrope of carbon.
The crystalline structure of diamond is FCC (face centered cubic), in which each carbon atom is bound to four other carbon atoms forming a triangular prism. Diamond occurs and exists only in mono-crystal form.
Properties of diamond are characterized by high hardness (the hardest of the natural minerals) and high refractive index 2.42 (measure of how a ray of light bends when passes from vacuum to the medium). Due to these characteristics diamond is widely used in jewelry. Industrial applications of diamond are associated with its hardness: cutting tools, grinding and polishing.
Natural diamonds of high optic quality are very rare and extremely expensive therefore significant efforts have been made to develop industrial techniques of producing artificial (synthetic) diamonds.
Synthetic diamonds have been fabricated commercially since 1950s.
Diamond types
The price of a diamond depends on its size (weight) and the quality (clarity, color, presence of inclusions).
The diamond color is determined by the impurities dissolved in the carbon structure.
According to the kind of impurity and its content the diamonds are classified by four types:
- I - diamonds containing a measurable amount of nitrogen.
- Ia - contain 500-3000 ppm of Nitrogen. Ia diamonds appear pale yellow (absorb blue light).
- Ib - contain up to 500 ppm of nitrogen. Ib diamonds have dark yellow-to-brown color since their structure absorbs not only blue but also green light.
- II - diamonds containing very low (unmeasurable) amount of nitrogen impurities.
- IIa - diamonds having very pure carbon structure. IIa diamonds are transparent and colorless.
- IIb - contain boron (B) and traces of nitrogen. Boron dissolved in the diamonds make them to appear light blue or grey (IIb diamonds absorb yellow, orange and red lights).
Fabrication of synthetic diamonds by High Pressure High Temperature (HPHT) method
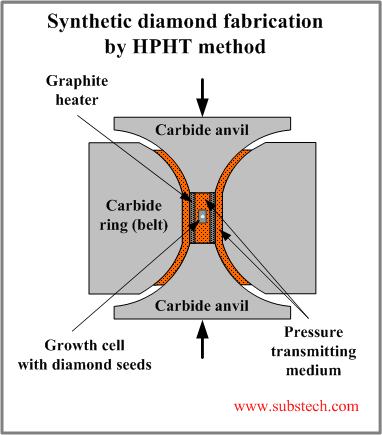 High Pressure High Temperature (HPHT) method produces conditions environment similar to those of diamond growth in Nature.
High Pressure High Temperature (HPHT) method produces conditions environment similar to those of diamond growth in Nature.
Tiny diamond seeds are placed into an equi-axially pressurized growth cell containing molten metal (iron, cobalt, nickel) with carbon atoms dissolved in it.
The combination of high pressure of about 725,000-800,000 psi (5-5.5 GPa) and high temperature from the range 2372-2732°F (1300-1500°C) causes the carbon atoms to deposit on the surface of the seeds.
The Ib crystals of 9 carats (about half inch/12 mm size) may be grown by HPHT method at a rate as high as 15 mg/hr.
High quality IIa diamonds of 8 carats (0.4”/10 mm size) were produced at the growth rate 7 mg/hr.
The scheme of a typical equipment for HPHT process is shown in the picture.
High pressure is created by the belt press. The upper and lower anvils produce a high load, which is transmitted to the growth cell through the pressure transmitting medium. Hydrostatic (equi-axial) character of pressure is achieved due to the ring (belt) surrounding the cell and confining it in radial direction.
High temperature is produced by the graphite heater.
to top
Fabrication of synthetic diamonds by Chemical Vapor Deposition (CVD) method
Chemical Vapor Deposition (CVD) technique utilizes deposition of carbon atoms on the substrate of diamond seeds from the gaseous phase (1-5% of carbon in Hydrogen).
A mixture of methane and hydrogen is fed to the CVD chamber where it is ionized by one of the following methods: hot filament, microwave plasma assisted CVD, radio frequency discharge, arcjet torch, electron beam, laser.
The pressure of the vapor phase is 3-4 psi (20.7-27.6 kPa). The temperature range is 1300-2400°F (704-1316°C).
High purity colorless IIa diamonds are produced by CVD technique with the rate as high as 0.6 mil/hr (15 µm/hr). Chemical Vapor Deposition is used not only for fabrication of high quality diamond single crystals but also for the deposition of polycrystalline diamond and amorphous Diamond-Like Carbon coatings.
The deposition rate of polycrystalline diamond coating may reach 6 mil/hr (150 µm/hr).
to top
Fabrication of synthetic diamonds by detonation
Detonation is the method similar to HPTP.
In the detonation (explosion) technique the high pressure is formed as a result of a shock wave passing through a carbon material commonly contained in the explosive.
The wave creates an extremely high pressure 2900-5000 ksi (20-35 GPa). The material is also instantly heated up to a temperature 5000-6200°F (2760-3427°C).
Under such conditions the carbon material transforms into tiny (1-5 nm) diamond single crystals. The crystals are not able to grow to larger dimensions because of very short duration of the high pressure conditions.
The diamond crystals produced by the detonation method are used as abrasive (polishing) material.
The process and conditions of fabrication synthetic diamond by the detonation method are similar to the formation of nanodiamonds as a result of a meteorite impact.
to top
Related internal links


