Main page
About us
Sliding Bearings Consulting
Advertising Opportunities

to Metals
to Coating technologies
Diffusion layer
Dr. Dmitri Kopeliovich
As Electroplating process proceeds the positively charged ions (of Metals or Hydrogen) are formed at the anode surface, transferred to the cathode and deposit on its surface when the negatively charged ions move toward the anode where they discharge.
Such ion transfer process results in formation of thin electrolyte regions (diffusion layers) adjacent to the electrodes where there are gradients of the ion concentration. The concentration beyond of the regions (bulk concentration) is constant with respect to the distance from the electrodes.
Within the cathodic diffusion layer the metal ion concentration non-linearly decreases toward the cathode surface.
At the anode surface where the metal dissolves in the electrolyte the ion concentration is higher than in the bulk solution.
Electrolyte convection caused by stirring (agitation) results in decrease of the diffusion layer.
Diffusion of the ions through the layers controls the material transfer and the deposition rate according to the First Fick's law: 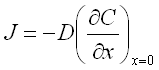
Where:
J - flux of the ions in x direction;
C - ion concentration as a function of x (the distance from the cathode surface);
D - diffusion coefficient of the ions in the electrolyte.
Diffusion layer causes concentration polarization of the electrochemical process.
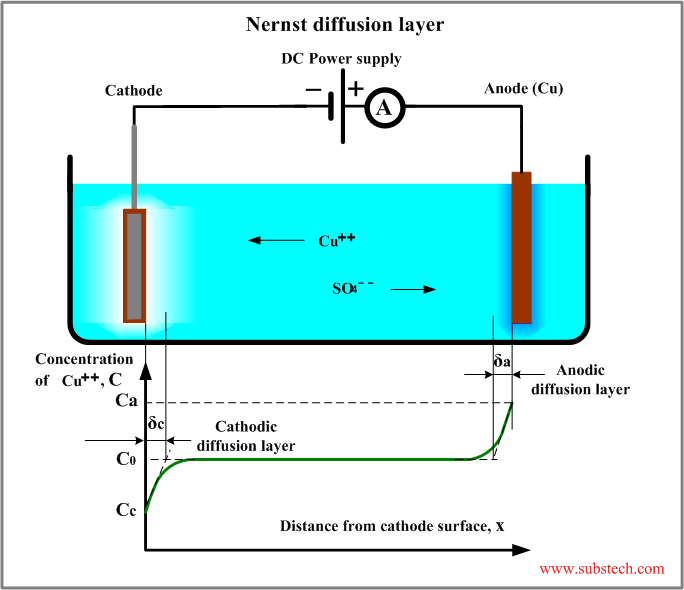
Nernst diffusion layer
Nernst diffusion layer is a virtual layer, within which the gradient of the ion concentration is constant and equal to the true gradient at the electrode-electrolyte interface.
The thickness of the Nernst diffusion layer may be measured on the graph “ion concentration vs. distance from the electrode surface”. The layer extends form the electrode surface to the point of intersection of the horizontal line corresponding the bulk concentration with the tangent to the curve at the interface.
Thickness of the Nernst diffusion layer varies within the range 0.1-0.001 mm depending on the intensity of convection caused by agitation of the electrodes or electrolyte.
According to the definition of the Nernst diffusion layer the concentration gradient may be determined as follows: 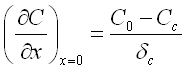
Where:
C0 - bulk concentration;
Cc - concentration of the ions at the cathode surface;
δc - thickness of the Nernst diffusion layer.
Therefore the flux of ions toward the cathode surface:
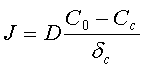 Each ion possesses an electric charge. The density of the electric current formed by the moving ions:
Each ion possesses an electric charge. The density of the electric current formed by the moving ions:
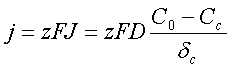 Where:
Where:
F - Faraday’s constant, F = 96485 Coulombs;
z - number of elementary charges transferred by each ion.
The maximum flux of the ions may be achieved when Cc=0 therefore the electric current density is limited by the value:
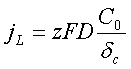 The value of the limiting current density may be increased by the following methods:
The value of the limiting current density may be increased by the following methods:
- Increase of the bulk ions concentration C0.
- Increase the electrolyte temperature, which strongly affect on the diffusion coefficient D.
- Intensification of agitation resulting in lowering the diffusion layer thickness δc.
Related internal links
Related external links


