Main page
About us
Sliding Bearings Consulting
Advertising Opportunities

Ammonia cracker
Dr. Dmitri Kopeliovich
Ammonia cracking is a process of dissociation of gaseous anhydrous Ammonia (NH3) into a mixture of Hydrogen (H2) and Nitrogen (N2) according to the reaction:
2NH3 = N2 + 3H2
The reaction is endothemic, requiring 383 kJ/mol. The process is performed at increased temperature within the range 1560-1740ºF (850-950ºC) in the presence of nickel as catalyst.
The resulting gas mixture is composed of hydrogen and nitrogen in the proportion 3:1 (75% of H2 and 25% of N2) with very little amount (20 -100 ppm) of residual undissociated ammonia with dew point -60ºF to -20ºF (-51ºC to -29ºC). The gas may be further purified by molecular sieves purifier resulting in reducing the uncracked ammonia to 1-3 ppm with dew point -110ºF (-80ºC).
The principal flow scheme of ammonia cracker is presented in the figure:
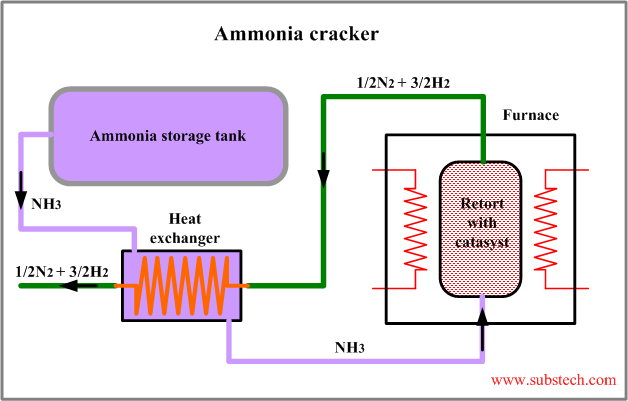
Ammonia cracking is a simple and cost effective method of manufacturing hydrogen, however it may be used only in the applications, in which presence of nitrogen is acceptable.
The main applications of ammonia cracker:
- Metal powder annealing.
- Hydrogenation of oil.
Related internal links


