Main page
About us
Sliding Bearings Consulting
Advertising Opportunities

to Metals
to Coating technologies
Anodizing
Dr. Dmitri Kopeliovich
Anodizing is an electrochemical process of growing conversion oxide coating as a result of oxidation of an anodically connected metal in an acidic electrolyte solution.
Conversion coating is a film of a chemical compound formed in the reaction of the substrate substance with another substance. This reaction distinguishes conversion coating from a conventional coating applied on the substrate surface without changing its chemical state. Examples of conversion coating are black oxide, which is formed on the metal surface as a result of a chemical reaction of the metal atoms with an oxidizing agent (air, aqueous solution, molten salts) and Phosphating (coating consisting of an insoluble crystalline metal-phosphate salt formed in a chemical reaction between the substrate metal and a phosphoric acid solution).
Anodizing is mostly used for aluminum.
Other commercially anodized metals are tantalum and niobium.
Electrochemistry of anodizing
Anodizing process occurs in an electrochemical cell, in which the anode is the anodized part and the cathode is a plate/rod made of a material chemically inert in the acidic electrolyte (carbon, Stainless steels, nickel).
Electrochemical reaction at the anode
2Al + 3H2O = Al2O3 + 6H+ + 6e-
Electrochemical reaction at the cathode
6H+ + 6e- = 3H2
Resulting anodizing reaction
2Al + 3H2O = Al2O3 + 3H2
In aqueous solutions aluminum oxide may form various hydrates
Al2O3*(H2O)n
n=1 to 3
to top
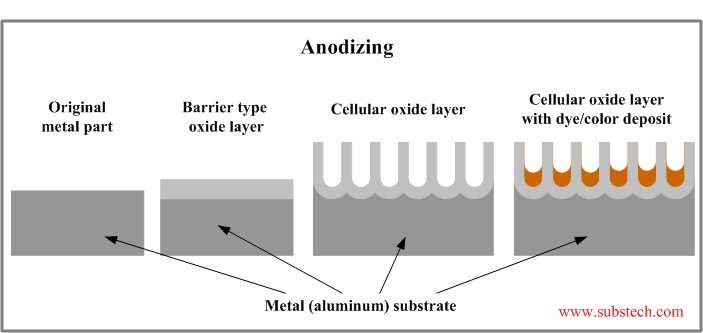
Oxide layer structure
Oxide coating formed as a result of anodizing may be in two forms: thin barrier-type layer or thick porous cellular layer.
The barrier-type oxide film forms in neutral electrolyte solutions (eg. ammonium borate), in which aluminum oxide does not dissolve. Thickness of barrier-type film is determined by the voltage applied between the anode and cathode. Maximum film thickness built at 700V is below 0.04 mil (1 µm).
The porous oxide film forms in acidic electrolyte solution (eg. diluted sulfuric acid), in which aluminum oxide not only grows but also dissolves.
Cellular (porous) structure of the oxide layer is a result of fluctuations on the metal surface causing local concentrations of the electric current and enhanced oxide film growing.
Thickness of the porous oxide layer may reach 4 mil (100 µm). Oxide coating is always thicker than the metal layer converted to oxide: 40% of the total oxide film penetrates below the original metal surface; 60% of the thickness is above the original metal surface.
The pore structure is very fine: their surface density is typically varies in the range 30-60*1010 cells/in² (50-100 cells/µm²).
to top
Clear anodizing
Clear anodizing is the anodizing process resulting in formation of translucent clear film.
Clear anodize coating is usually up to 0.001” (25 µm) thickness. It is produced in sulfuric acid solutions and then sealed in hot water.
Clear anodizing provides good corrosion resistance and moderate wear resistance.
to top
Hard anodizing
Hard anodizing is the anodizing process resulting in formation of high density coating with large cells and small pores. It is produced in sulfuric acid at low temperature.
Hard anodize coating is extremely durable and abrasion/wear resistant.
to top
Dying
Dying is a process of absorption of organic or inorganic molecules in the pores of an anodize coating.
The dye fills the microscopic pores and imparts the coating a color.
The dyed coating is usually sealed in hot water. Dying provides excellent decorative appearance to the anodized part.
to top
Electrolytic coloring
Electrolytic (2-step) coloring is a process of Clear anodizing followed by AC deposition of a metal (commonly tin or nickel) onto the bottom of the oxide pores.
Electrolytic coating produces bronze color due to optical interference caused by the metal deposition. Electrolytic (2-step) coating possess stable and durable color.
to top
Sealing
Sealing of an anodize oxide coating is performed in order to trap the dye locating in the pores and prevent absorption of undesired molecules in the pores. Sealing is conducted in hot water at a temperature about 200ºF (93ºC). Crystals of hydrated aluminum oxide form in hot water. The crystals seal the pores opening. Sealing may also be provided by deposition of metal salts (nickel acetate) dissolved in hot water at 180ºF (86ºC). Anodize coatings used for application of paints and adhesives are not sealed.
to top
Stages of anodizing process
- Racking - connection of the part to a system providing electrical connection of the part and its transportation through the baths of the anodizing line.
- Surface preparation - a series of cleaning treatments (alkaline cleaning, etching by sodium hydroxide, desmutting in acid) of the substrate surface prior to the anodizing operation, intended for ensuring strong and uniform adhesion of the coating to the substrate.
- Anodizing in 10% sulfuric acid solution by DC current of density 10-20 A/ft² (1-2 A/dm²) for 10-60 min.
- Unracking.
- Inspection.
Related internal links
to Metals
to Coating technologies


