Fundamentals of adhesive bonding
Dr. Dmitri Kopeliovich
Adhesive bonding is a process of joining two or more solid parts with an adhesive substance.
The materials of the joined parts (adherents, substrates) may be different or similar.
The material of the adhesive layer is commonly a polymer (natural or synthetic). Thickness of the adhesive layer does not usually exceed 0.02” (0.5 mm).
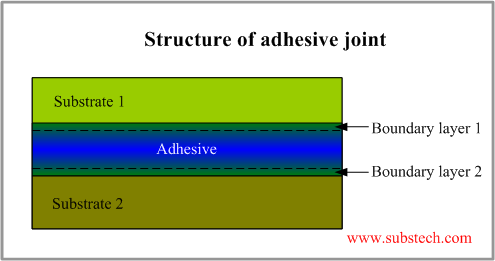
Structure of adhesive joint
Adhesive joint generally consists of two substrate surfaces with the adhesive material filled the gap between them. However the adhesive layer is not uniform. Besides the part of the adhesive layer, properties of which are not affected by the substrate, there are two boundary layers, which have been changed by impurities and products of reactions at the substrate surfaces.
Boundary layer is a part of the adhesive layer adjacent to the substrate surface.
Adhesion
The principle basis of adhesive bonding is a phenomenon called adhesion
Adhesion is a complex of physicochemical processes occurring at the interface of two materials brought into an intimate contact, which result in formation of an attractive force between the two materials.
Adhesion strength is a force required for separation of two adhered parts along the interface.
The following factors determine the value of adhesion strength of adhesive bonding:
- Mechanical factor
Porous or roughened surfaces provide stronger adhesion due to:
a. larger interfacial area;
b. interlocking the adhesive material in the surface micro-voids.
- Chemical bonding
Molecules of the adherent material may form chemical bonds with the molecules of the adhesive across the interface.
The chemical bonds may be either ionic or covalent.
Ionic bond is formed when an atom donates its electron to another atom. As a result of the electron transition two ions form: positively charged cation and negatively charged anion. The force of electrostatic attraction between the two ions forms ionic bond. Ionic bond may be formed between two materials with different electronegativities (the difference is grater than 1.7).
Covalent bond is a chemical bond, in which two atoms share one or more pairs of electrons. Covalent bond may be formed between two materials with similar electronegativities (the difference is lower than 1.7).
Metallic bond is a chemical bond, in which each of the atoms of the metal contributes its valence electrons to the crystal lattice, forming an electron cloud or electron “gas”, surrounding positive metal ions. These free electrons belong to the whole metal crystal and hold together the atoms of a metal.
In practice most adhesive materials are polymeric therefore metallic bonds do not form in adhesive joints.
- Intermolecular bonding
Intermolecular bonds are result of relatively weak attraction forces between the neighboring molecules.
Hydrogen bonding is an intermolecular bonding formed between a hydrogen atom chemically bonded to an electronegative atom and other electronegative atom of a neighboring molecule.
Van der Waals forces are the result of electrostatic attraction between neighboring molecules having permanent or instant transient dipole groups.
- Diffusion
Diffusion may become an important factor of an adhesive bonding if the adhesive and adherent (substrate) materials are similar and their molecules are mobile enough and capable to move across the interface.
to top
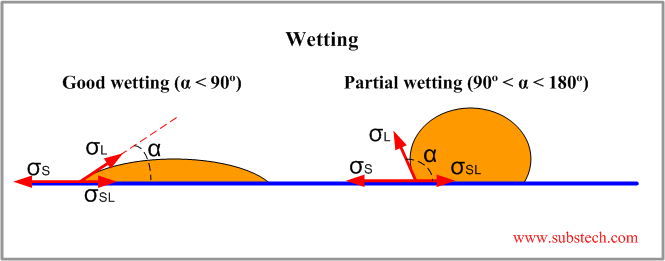
Wetting
Wetting the adherents surfaces with the liquid adhesive is necessary for formation of strong adhesion.
Wetting is a process of spreading a drop of a liquid over the surface of solid substrate.
The forces of surface tension applied to a drop on a substrate are shown in the figure:
σS = σL*cosα + σSL
cosα = (σS - σSL)/σL
Where:
σS - surface tension of the solid substrate;
σL - surface tension of the liquid adhesive;
σSL - intefacial surface tension between the liquid adhesive and solid substrate;
α - wetting angle.
Wetting angle characterizes wetting ability of the adhesive on the substrate surface. Good wetting and consequently good spreading of the adhesive are achieved at low values of α (α < 90°) when σSL < σS.
Partial wetting and non-wetting conditions are characterized by high values of α (90° < α < 180°).
to top
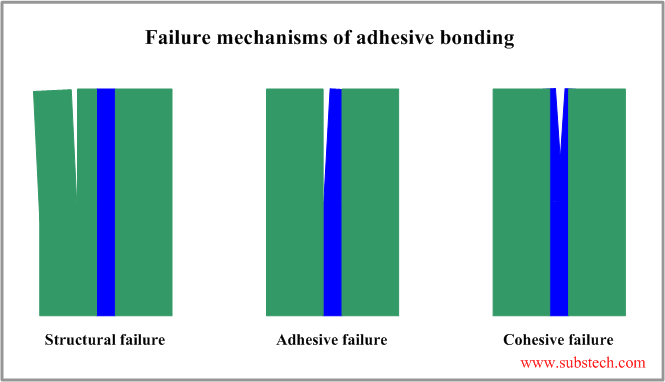
Failure of adhesive bonding
There are three possible mechanisms of failure of adhesive bonding:
- Structural failure - internal failure of a substrate material in a region close to the joint.
- Adhesive failure - interfacial failure resulted in separation of one of the substrate from the adhesive layer.
- Cohesive failure - internal failure of the adhesive layer.
Mechanism of cohesive failure is determined by cohesion - internal intermolecular attraction force holding the material in a united state.
to top