to Metals
to Coating technologies
Electropolishing
Principle of electropolishing
Electropolishing is an electrochemical process in which the atoms of a work piece submerged in an electrolyte convert into ions and are removed from the surface as a result of a passage of an electric current.
In electropolishing the metallic work piece dissolves in the electrolyte in contrast to Electroplating where the metallic ions traveling through the electrolyte solution deposit on the work piece surface.
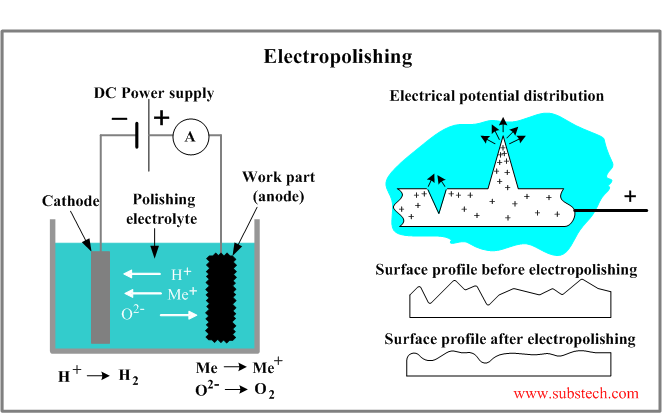
In an electropolishing cell (see the figure below) the work piece is anode. It is connected to the positive terminal of the direct current power supply. The negative terminal is connected to a cathode plate commonly made of stainless steel, copper or lead. The anode and the cathode are immersed into an electrolyte solution.
Principally electropolishing is similar to Electrochemical machining where the work piece material is removed due to the conversion of the atoms into ions, formation of an insoluble precipitate and its transfer from the surface. The DC power supply, the anodically connected work piece, the electrolyte and the cathode form an electric circuit.
According to Faraday’s law the amount of the removed material is directly proportional to the amount of electric charge, passed through the circuit. The amount of electric charge Q=I*t (I – electric current, t – time).
The amount of the metal removed from the work piece surface in an electropolishing process varies from 0.1 to 2.5 mil (2.5-64 μm).
The important feature of the electropolishing process is its ability to dissolve asperities (peaks) on the work surface much faster than the material in “micro-valleys”. Such selective dissolution is a result of different values of the electrical potential of the peaks and valleys. The positive charge of the anodically connected work piece is concentrated in the peaks where the current density is higher than average which causes a selective dissolution of the peaks and smoothening the surface.
to top
Brightening
Brightening is an effect of lower surface roughness produced by electropolishing operation.
The surface of the parts produced by metalworking operations (cutting, Rolling, stamping, Drawing) is relatively rough and has to be improved by a polishing operation. One of the polishing techniques is a mechanical polishing. However mechanical polishing produces scratches, debris and may leave the abrasive particles on the work surface. Additionally mechanical polishing causes a formation of residual stresses in the surface layer.
In contrast to mechanical polishing electropolishing produces a surface free of both mechanical defects and residual stresses.
Surface roughness is a parameter of the surface quality commonly measured as the average deviation of the surface profile from the mean line.
Electropolishing reduces the profile peaks resulting in smoothening the surface.
The improvement of the surface roughness achieved by electropolishing is commonly a half of the original roughness value.
to top
Deburring
The selective dissolution of prominent point (peaks) on the work surface in the electropolishing process is utilized in deburring operation.
Small burrs (up to 0.5 mil/13 μm) produced in some various metalworking operations may be effectively removed by electropolishing.
Like in the peaks on the surface the density of the electric current passing through burrs is higher than the average value therefore the burrs material is removed much faster. The resulting surface is electropolished and free of burrs.
In contrast to mechanical deburring (e.g. vibratory deburring) electropolishing does not produce scratches and residual stresses on the work surface.
to top
Passivating
Passivation is a chemical process of a restoration of the corrosion resistance of a contaminated stainless steel part.
The contaminant particles embedded into the surface disturb the protecting layer of chromium oxide and allow oxidation of iron (rust formation).
In the conventional passivation process the contaminants are removed by a treatment in 20% nitric acid.
The contaminating particles may also be removed by electropolishing. Additionally electropolishing produces higher surface concentration of chromium due to the preferential dissolution of iron and nickel atoms.
In contrast to the treatment by nitric acid the passivation by electropolishing does not produce distortion and does not cause hydrogen embrittlement.
to top
Stress relieving
Tensile stresses concentrated in the part surface reduce its Fatigue.
The stresses may be induced in various fabrication stages: metalworking operations (stamping, Rolling, Drawing, grinding), heat treatment, vibratory deburring, Electroplating.
The thickness of the layer with the surface stresses is about 1 mil (25 μm).
Electropolishing allows to remove the stressed surface skin from the work piece.
The fatigue strength is additionally improved due to smoothening the surface of electropolishing. The surface defects such as scratches, foreign particles embeded into the surface, tool produce notch effect decreasing the fatigue limit.
Defects free electropolished surface provides an increased fatigue strength.
to top
Benefits and disadvantages of electropolishing
Benefits of electropolishing:
- Bright appearance;
- Absence of abrasive scratches;
- Improved fatigue strength due to stress relieving and defects free surface;
- Lower coefficient of friction due to smoother surface (reduced microasperities);
- Better corrosion resistance;
- Allows processing fragile and delicate parts.
Disadvantages of electropolishing:
- Rough surface defects can not be removed;
- Electropolishing multiphase alloys may cause roughening due to selective dissolution of different phases.
Related internal links
to Metals
to Coating technologies