Main page
About us
Sliding Bearings Consulting
Advertising Opportunities

to Fluids
to Dispersions
Stabilization of colloids
Dr. Dmitri Kopeliovich
Colloidal particles collide with each other due to the Brownian motion, convection, gravity and other forces.
Collisions may result in coagulation of the particles and destabilization of the colloid.
If a colloidal particle is brought to a short distance to another particle, they are attracted to each other by the van der Waals force. If there is no counteracting force, the particles will aggregate and the colloidal system will be destabilized.
Colloidal stability is achieved due to repulsion forces balancing the attraction forces in the way similar to the stable mechanic equilibrium {if a body is disturbed it tends to return to its former position).
According to the kind of the repulsion force two mechanisms of the colloidal stability take place:
Electrostatic stabilization of colloids
Electrostatic stabilization of Colloids is the mechanism in which the attraction van der Waals forces are counterbalanced by the repulsive Coulomb forces acting between the negatively charged colloidal particles.
Electric Double Layer
Electric Double Layer is the phenomenon playing a fundamental role in the mechanism of the electrostatic stabilization of colloids.
Colloidal particles gain negative electric charge when negatively charged ions of the dispersion medium are adsorbed on the particles surface.
A negatively charged particle attracts the positive counterions surrounding the particle.
Electric Double Layer is the layer surrounding a particle of the dispersed phase and including the ions adsorbed on the particle surface and a film of the countercharged dispersion medium.
The Electric Double Layer is electrically neutral.
An Electric Double Layer consists of three parts:
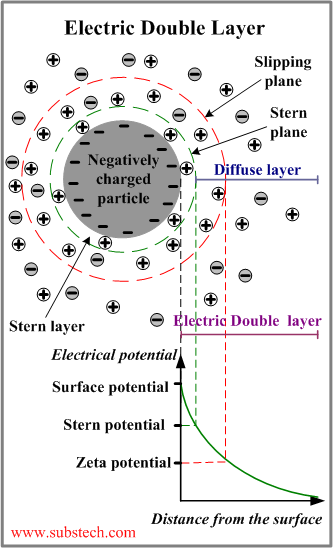
- Surface charge - charged ions (commonly negative) adsorbed on the particle surface.
- Stern layer - counterions (charged opposite to the surface charge) attracted to the particle surface and closely attached to it by the electrostatic force.
- Diffuse layer - a film of the dispersion medium (solvent) adjacent to the particle. Diffuse layer contains free ions with a higher concentration of the counterions. The ions of the diffuse layer are affected by the electrostatic force of the charged particle.
The electrical potential within the Electric Double Layer has the maximum value on the particle surface (Stern layer). The potential drops with the increase of distance from the surface and reaches 0 at the boundary of the Electric Double Layer.
When a colloidal particle moves in the dispersion medium, a layer of the surrounding liquid remains attached to the particle. The boundary of this layer is called slipping plane (shear plane).
The value of the electric potential at the slipping plane is called Zeta potential, which is very important parameter in the theory of interaction of colloidal particles.
to top
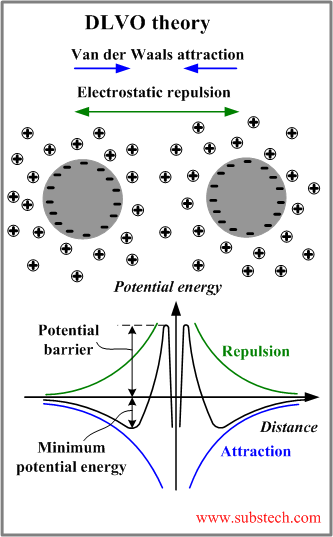
DLVO theory
In 1940s Deryagin, Landau, Vewey and Overbeek developed a theory of the stability of colloidal systems (DLVO theory).
Assumptions of DLVO theory:
- Dispersion is dilute
- Only two forces act on the dispersed particles: van der Waals force and electrostatic force
- The electric charge and other properties are uniformly distributed over the solid surface
- The distribution of the ions is determined by the electrostatic force, Brownian motion and entropic dispersion.
DLVO theory provides good explanation of the interaction between two approaching particles.
The theory states that the colloidal stability is determined by the potential energy of the particles (VT) summarizing two parts: potential energy of the attractive interaction due to van der Waals force VA and potential energy of the repulsive electrostatic interaction VR:
 VT = VA + VR
VT = VA + VR
For the spherical particles:
Van der Waals attractive potential energy:
VA = - Ar/(12x)
where:
A - Hamaker constant;
r - radius of the particles;
x - distance between the surfaces.
Electric repulsive potential energy:
VR = 2πεε0rζ2e-kx
where:
ε - dielectric constant of the solvent;
ε0 - vacuum permittivity;
ζ - zeta potential;
k - a function of the ionic concentration (k-1 is the characteristic length of the Electric Double Layer).
The graphs describing the potential energy of the interaction between two particles are presented in the figure above.
The minimum of the potential energy determines the distance between two particles corresponding to their stable equilibrium. The two particles form a loose aggregate, which can be easily re-dispersed. The strong aggregate may be formed at a shorter distance corresponding to the primary minimum of the potential energy (not shown in the picture). In order to approach to the distance of the primary minimum the particle should overcome the potential barrier.
to top
Polymeric stabilization of colloids
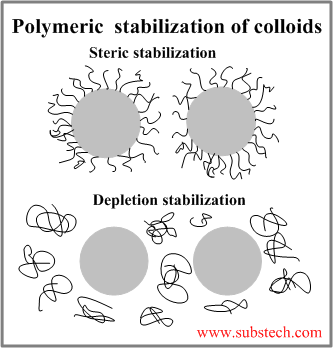 Polymeric stabilization of Colloids involves polymeric molecules added to the dispersion medium in order to prevent the aggregation of the colloidal particles.
Polymeric stabilization of Colloids involves polymeric molecules added to the dispersion medium in order to prevent the aggregation of the colloidal particles.
The polymeric molecules create a repulsive force counterbalancing the attractive van der Waals force acting on a particle approaching another particle.
There are two types of polymeric stabilization:
- Steric stabilization of colloids is achieved by polymer molecules attached to the particle surface and forming a coating, which creates a repulsive force and separates the particle from another particle.
- Depletion stabilization of colloids involves unanchored (free) polymeric molecules creating repulsive forces between the approaching particles.
Related internal links


