Main page
About us
Sliding Bearings Consulting
Advertising Opportunities

to Metals
to Extractive metallurgy
Extractive metallurgy of magnesium
Dr. Dmitri Kopeliovich
Magnesium is the sixth most abundant element in the earth’s crust.
The main sources of magnesium compounds are:
- seawater (magnesium chloride, MgCl2)
and minerals:
- dolomite (CaCO3·MgCO3),
- magnesite (MgCO3),
- carnallite (KCl·MgCl2·6H2O).
There are two principal magnesium extraction processes:
Silicotermic process (Pidgeon, Magnetherm, Bolzano)
The process involves reducing molten magnesium oxide slag by ferrosilicon under low gas pressure at a temperature of about 2500F (1400ºC).
The metallic magnesium, formed in the process, evaporates and then condensates away from the hot region.
The condensed magnesium, having purity of 99,95% is then remelt and cast.
to top
Electrolytic process
The first stage of the process is a preparation of magnesium chloride feed which is followed by the electrolytic dissociation of magnesium chloride.
Industrial cell feeds consist of a mixture of dehydrated magnesium chloride, partly dehydrated magnesium chloride or dehydrated carnallite.
Dehydrated magnesium chloride is prepared by one of two methods: chlorination of magnesium oxide (IG Farben process) or dehydration of magnesium chloride brines.
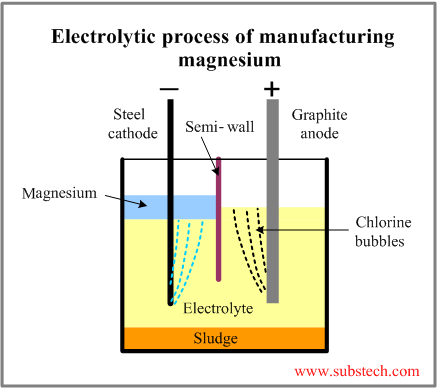
The electrolytic cell consists of a brick-lined vessel, divided into anode and cathode compartments by a semi-wall. Air- or water-cooled Graphite plate anode and steel cathode are submerged in electrolyte composed of alkaline chlorides with addition of magnesium chloride.
The operating temperature is 1260°F to 1380°F (680°C to 750°C).
Magnesium chloride decomposes in the electrolytic cell according to the reaction:
MgCl2→ Mg + Cl2(g)
Metallic magnesium is formed at the cathode. It floats up (it is lighter than electrolyte) collecting in the cathode compartment.
Chlorine, which is by-product of the process, is collected in the anode compartment.
to top
Related internal links