Main page
About us
Sliding Bearings Consulting
Advertising Opportunities

to Metals
to Coating technologies
Black copper oxide coating
Dr. Dmitri Kopeliovich
Black copper oxide is a conversion coating of cupric oxide (CuO) formed on the surface of a copper alloy as a result of a chemical reaction of copper atoms with an oxidizing agent (air, salts).
Conversion coating is a film of a chemical compound formed in the reaction of the substrate substance with another substance. This reaction distinguishes conversion coating from a conventional coating applied on the substrate surface without changing its chemical state.
Examples of conversion coating are black oxide on steel, Anodizing (electrochemical process of growing oxide film on the surface of anodically connected metal in an acidic electrolyte solution) and Phosphating (coating consisting of an insoluble crystalline metal-phosphate salt formed in a chemical reaction between the substrate metal and a phosphoric acid solution).
 Copper oxide coating is applied on the surface of a copper part for the purpose of either cosmetic coloring or as an adhesion promoter for a polymer overlay.
Copper oxide coating is applied on the surface of a copper part for the purpose of either cosmetic coloring or as an adhesion promoter for a polymer overlay.
A deep black cupric oxide is formed on the copper substrate prior to depositing a polymer coating (painting, lacquering or enamelling).
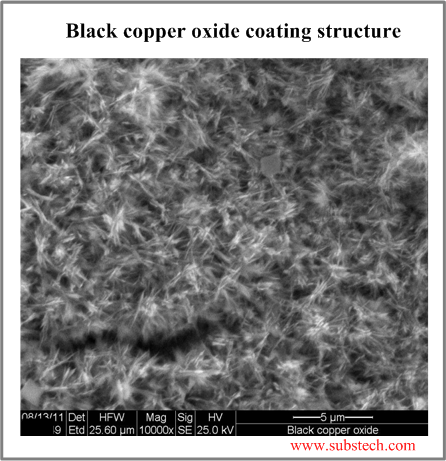
The oxide layer has a fibrous microstructure providing excellent adhesion of the polymer.
The black oxide coating consists of numerous dendrites of copper oxide crystals significantly increasing the bonding area between the copper surface and the polymer. Due to such structure the black copper oxide coating has a velevet appearance.
When a liquid resin is applied over the substrate surface it penetrates into the spaces between the dendrites. During the curing process the polymer is interlocked (anchored) in the oxide structure, which results in a strong adhesion.
Oxidizing (blackening, coloring) of a copper workpiece is commonly carried out by immersion of the part into a heated alkaline solution containing an oxidizing compound (e.g. sodium chlorite).
Prior to black oxide treatment the copper substrate is cleaned and acid activated.
Processes of blackening (coloring) copper:
- Bath composition: 13-26 oz/gal (100-200 g/l) of the mixture: sodium hydroxide (NaOH) 50%-67% and sodium chlorite (NaClO2) 50%-33%.
Blackening bath temperature: 210-215ºF (99-102ºC). Treatment time: 5-10 min.
Coloring bath temperature: 120-180ºF (49-82ºC). Treatment time: 5-60 sec.
A wide range of colors (from light to deep black) may be obtained by the oxidizing surface treatment of a copper/copper alloy part.
The higher the treatment temperature and greater its time the darker the resulting oxide layer is formed on the copper surface.
- Bath composition: trisodium phosphate (Na2PO4) 13 oz/gal (100 g/l), sodium hydroxide (NaOH) 6.6 oz/gal (50 g/l), sodium chlorite (NaClO2) 4 oz/gal (30 g/l).
Treatment temperature: 203ºF (95ºC). Treatment time: 15 min.
- Bath composition: sodium chlorite (NaClO2) 4-12 oz/gal (30-90 g/l), sodium hydroxide (NaOH) 1.3-2.7 oz/gal (10-20 g/l), trisodium phosphate (Na2PO4) 0.67-2 oz/gal (5-15 g/l).
Treatment temperature: 140-203ºF (60-95ºC). Treatment time: 2-5 min.
- Anodizing in a solution of sodium hydroxide (NaOH) 21 oz/gal (160 g/l).
Treatment temperature: 194ºF (90ºC).
Electric current density: 1.43 A/dec2.
to top
Related internal links
to Metals
to Coating technologies


