to Ceramics
to Fundamentals of ceramics
Ionic and covalent bonding
Dr. Dmitri Kopeliovich
Ceramics (ceramic materials) are non-metallic inorganic compounds formed from metallic (Al, Mg, Na, Ti, W) or semi-metallic (Si, B) and non-metallic (O, N, C) elements.
Atoms of the elements are held together in a ceramic structure by one of the following bonding mechanism: Ionic Bonding, Covalent Bonding, Mixed Bonding (Ionic-Covalent).
Most of ceramic materials have a mixed bonding structure with various ratios between Ionic and Covalent components. This ratio is dependent on the difference in the electronegativities of the elements and determines which of the bonding mechanisms is dominating ionic or covalent.
Electronegativity
Electronegativity is an ability of atoms of the element to attract electrons of atoms of another element. Electronegativity is measured in a relative dimensionless unit (Pauling scale) varying in a range between 0.7 (francium) to 3.98 (fluorine).
Non-metallic elements are strongly electronegative. Metallic elements are characterized by low electronegativity or high electropositivity – ability of the element to lose electrons.
to top
Ionic Bonding
Ionic bonding occurs between two elements with a large difference in their electronegativities (metallic and non-metallic), which become ions (negative and positive) as a result of transfer of the valence electron from the element with low electronegativity to the element with high electronegativity.
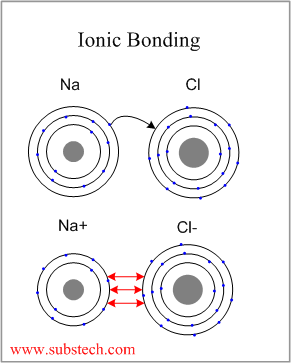
The typical example of a material with Ionic Bonding is sodium chloride (NaCl).
Electropositive sodium atom donates its valence electron to the electronegative chlorine atom, completing its outer electron level (eight electrons):
As a result of the electron transfer the sodium atom becomes a positively charged ion (cation) and the chlorine atom becomes a negatively charged ion (anion). The two ions attract to each other by Coulomb force, forming a compound (sodium chloride) with ionic bonding.
Ionic bonding is non-directional.
to top
Covalent Bonding
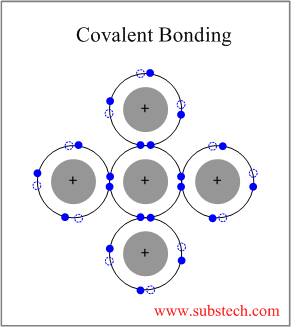
Covalent bonding occurs between two elements with low difference in their electronegativities (usually non-metallics), outer electrons of which are shared between the four neighboring atoms.
Covalent Bonding is strongly directional.
to top
Ionic-Covalent (mixed) Bonding
Ionic-covalent (mixed) bonding with various ratios of the two fractions (ionic and covalent) occurs in most of ceramic materials.
Degree of Ionic Bonding can be estimated from the following formula:
I.F. = exp(-0.25*ΔE²)
Where
I.F. – fraction of ionic bonding;
ΔE – difference in the electronegativities of the elements.
to top
Characterization of ceramics properties
In contrast to metallic bonding neither ionic nor covalent bonding form free electrons, therefore ceramic materials have very low electric conductivity and thermal conductivity.
Since both ionic and covalent bonds are stronger than metallic bond, ceramic materials are stronger and harder than metals.
Strength of ionic and covalent bonds also determines high melting point, modulus of elasticity (rigidity), temperature and chemical stability of ceramic materials.
Motion of dislocations through a ceramic structure is impeded therefore ceramics are generally brittle that limits their use as structural materials.
Ceramics may have either crystalline or amorphous structure. There are also ceramic materials, consisting of two constituents: crystalline and amorphous.
to top