to Metals
to Extractive metallurgy
Extractive metallurgy of magnesium
Dr. Dmitri Kopeliovich
Magnesium is the sixth most abundant element in the earth’s crust.
The main sources of magnesium compounds are:
- seawater (magnesium chloride, MgCl2)
and minerals:
- dolomite (CaCO3·MgCO3),
- magnesite (MgCO3),
- carnallite (KCl·MgCl2·6H2O).
There are two principal magnesium extraction processes:
Silicotermic process (Pidgeon, Magnetherm, Bolzano)
The process involves reducing molten magnesium oxide slag by ferrosilicon under low gas pressure at a temperature of about 2500F (1400ºC).
The metallic magnesium, formed in the process, evaporates and then condensates away from the hot region.
The condensed magnesium, having purity of 99,95% is then remelt and cast.
to top
Electrolytic process
The first stage of the process is a preparation of magnesium chloride feed which is followed by the electrolytic dissociation of magnesium chloride.
Industrial cell feeds consist of a mixture of dehydrated magnesium chloride, partly dehydrated magnesium chloride or dehydrated carnallite.
Dehydrated magnesium chloride is prepared by one of two methods: chlorination of magnesium oxide (IG Farben process) or dehydration of magnesium chloride brines.
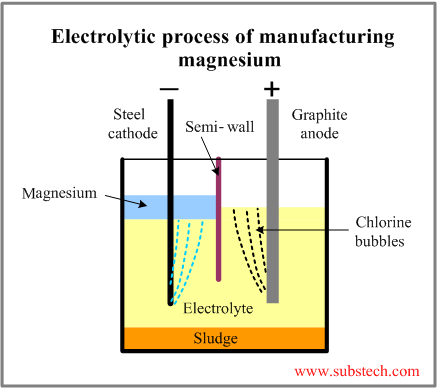
The electrolytic cell consists of a brick-lined vessel, divided into anode and cathode compartments by a semi-wall. Air- or water-cooled Graphite plate anode and steel cathode are submerged in electrolyte composed of alkaline chlorides with addition of magnesium chloride.
The operating temperature is 1260°F to 1380°F (680°C to 750°C).
Magnesium chloride decomposes in the electrolytic cell according to the reaction:
MgCl2→ Mg + Cl2(g)
Metallic magnesium is formed at the cathode. It floats up (it is lighter than electrolyte) collecting in the cathode compartment.
Chlorine, which is by-product of the process, is collected in the anode compartment.
to top