to Metals
to Extractive metallurgy
Extractive metallurgy of iron
Dr. Dmitri Kopeliovich
The following raw materials are involved in manufacturing iron:
- Iron ores (magnetite, hematite) – iron oxides with earth impurities;
- Coke, which is both reducing agent and fuel, providing heat for melting the metal and slag.
Coke is produced from coking coals by heating them away from air.
- Limestone – calcium silicate fluxes, forming a fluid slag for removal gangue from the ore.
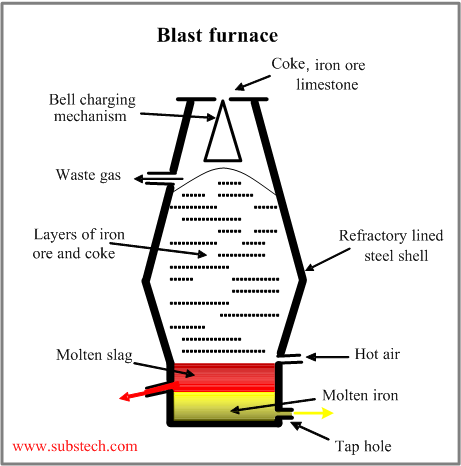
Iron is produced in a blast furnace, schematically shown in the picture.
It the shaft-type furnace consisting of a steel shell lined with refractory bricks.
The top of the furnace is equipped with the bell-like or other system, providing correct charging and distribution of the raw materials (ore, coke, limestone).
Air heated to 2200°F (1200°C) is blown through the tuyeres at the bottom.
Oxigen containing in air reacts with the coke, producing carbon monoxide:
2C + O2= 2CO
Hot gases pass up through the descending materials, causing reduction of the iron oxides to iron according to the follwing reactions:
3Fe2O3 + CO = 2Fe3O4 + CO2
Fe3O4 + CO = 3FeO + CO2
FeO + CO = Fe + CO2
Iron in form of a spongy mass moves down and its temperature reaches the melting point at the bottom regions of the furnace where it melts and accumulates.
The gangue, ash and other fractions of ore and coke are mixed by fluxes, formig slag which is capable to absorb sulphure and other impurities.
The furnace is periodically tapped andthe melt (pig iron) is poured into ladles, which are transferred to steel making furnaces.
Pig iron usually contains 3-4% of carbon, 2-4% of silicon, 1-2% of manganese and 1-1.2% of phosphorous.
to top