to Metals
to Extractive metallurgy
Extractive metallurgy of aluminum
Dr. Dmitri Kopeliovich
Aluminum is the most abundant metal in Earth.
It occurs in the nature in form of aluminum oxide and other combined forms.
The ore containing aluminum compound, which is commercially used in the extractive metallurgy is called bauxite.
Bauxite is a hydrated aluminum oxide.
Extraction of aluminum from bauxite is carried out in three stages:
- Ore dressing– cleaning ore by means of separation of the metal containing mineral from the waste (gangue).
- Chemical treatment of bauxite (Bayer process) for converting the hydrated aluminum oxide to pure aluminum oxide (Al2O3).
At this stage crushed and ground bauxite is mixed with hot sodium hydroxide solution, which dissolves the aluminum hydroxide, forming solution of sodium aluminate.
The residual impurities (oxides of silicon, iron and titanium), which called “red mud”, are separated from the sodium aluminate solution.
The solution is then treated in precipitator tanks, where aluminum trihydrate precipitates from the solution.
The aluminum trihydrate after separation from the sodium hydroxide is converted into pure aluminum oxide by heating to 1800F (1000ºC).
- Reduction of aluminum from aluminum oxide by the electrolytic process.
As aluminum oxide is a very poor electricity conductor its electrolysis is carried out in a bath of molten cryolite (mineral, containing sodium aluminum fluoride – Na3AlF6).
The technology is called Hall-Heroult process.
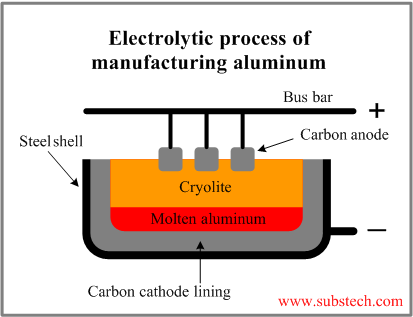
Schematically the process is presented in the picture:
The electrolytic cell for aluminum production consists of a pot with carbon lining, serving as negative electrode (cathode) and positive electrodes (anodes), connected to the current conductor (bus bar).
The anodes are immersed into the bath of molten cryolite.
The aluminum oxide is added to the cryolite and dissolved in it. When electric current passes between the anodes and the cathode through the cryolite, aluminum oxide decomposes to metallic aluminum deposited at the cathode and oxygen liberated at the anode.
The molten aluminum is periodically tapped from the furnace into a crucible and cast into ingots.
to top