to Metals
to Coating technologies
Electrophoretic deposition
Principle of electrophoretic deposition
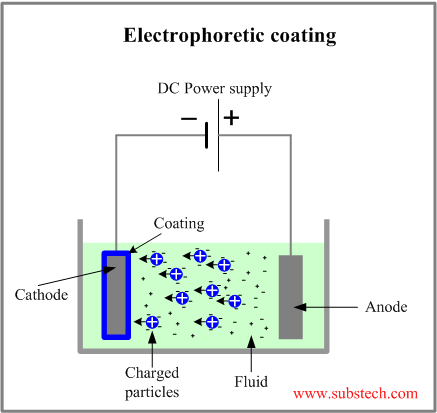 Electrophoretic deposition EPD is a method of coating a conductive part with particles suspended in a fluid dispersion under the influence of an electric field applied between the work part and the counter electrode.
Electrophoretic deposition EPD is a method of coating a conductive part with particles suspended in a fluid dispersion under the influence of an electric field applied between the work part and the counter electrode.
Similar to Electroplating coating electrophoretic deposition utilizes electrically charged particles moving between two electrodes (an anode and a cathode) immersed in a liquid media.
However in contrast to conductive electrolytes used in electroplating, the fluids of electrophoretic dispersions are dielectric.
In addition the electroplating coatings are built from metallic ions converted into atoms when discharged at the cathode, whereas in electrophoretic process the coating is formed by a deposition of relatively large powder particles which may be polymeric, ceramic or metallic.
The following characteristic features define electrophoretic deposition:
- There is a stable dispersion of particles in a solvent (a colloid).
- The particles gain a surface charge as a result of an electrostatic interaction with the molecules of the solvent.
- The particles are capable to move in the suspension towards the work part under the influence of a voltage imposed between the work part and the counter electrode (the phenomenon called electrophoresis).
- A rigid deposition composed of condensed particles is built up on the work part surface in contrast to dip coating - a painting process in which the concentration of particles in the layer adhered to the work part is the same as the concentration of the particles in the bulk suspension.
- The deposited coating is adhered to the surface of the work part.
When a voltage is applied between the electrophoretic electrodes the charged particles start to migrate towards the electrode with the opposite electric polarity. The particles are deposited first of all at the surface areas with the highest electric potential. The formed coating decreases the potential of such areas and equalizes the potential distribution over the part surface. As a result a uniform and even film forms over the whole part surface including the surface of cavities, edges and corners.
The coating thickness is determined by the voltage value which is typically 25-400 V. After a coating of a certain thickness is built the deposition process stops.
If the electrophoretic dispersion is aqueous, the voltage applied between the immersed electrodes immersed causes water molecules to decompose.
At the anode Oxygen is generated according to the oxidation reaction:
2 H2O = O2 + 4 H+ + 4e-
The reduction reaction at the cathode results in a formation of Hydrogen:
2 H2O + 2e- = H2 + 2 OH-
As a result of electrolysis the solution surrounding the anode becomes acidic and in the cathode region - alkaline.
Changes of the solution PH destabilize the colloid causing the particles to coagulate and deposit on the electrode surface.
to top
Polarity of electrophoretic deposition
The electric charge of the dispersed particles may be either positive or negative.
The positively charged particles are attracted to the cathode. The process of a deposition of positively charged particles is classified as cathodic electrophoretic coating in contrast to anodic electrophoretic coating related to the deposition of negatively charged particles.
Anodic electrophoretic process is less expensive. It produces coatings of good aesthetic appearance. However the anode metal slightly dissolves in the solvent producing metallic ions which are incorporated in the coated film. The metallic contamination of anodic coatings decreases their corrosion resistance. Therefore anodic electrophoretic coatings are mostly used in indoor applications.
Cathodic electrophoretic process results in a deposition of coatings with very low metallic contamination. Cathodic coatings are characterized by high corrosion resistance and durability in both indoor and outdoor applications.
to top
Electrophoretic coating process
The industrial applications of electrophoretic deposition are referred to as e-coating, electrocoating, electrophoretic painting or electrophoretic coating.
The deposition process includes the following stages:
- Hanging the work parts
- Immersion in adhesion promoter (wetting agent)
- Electrphoretic deposition
- Rinsing and dispersion recovery
- Rinsing in de-ionized water
- Dehydration oven
- Curing
The most popular applications of electrophoretic deposition are cathodic coating of automotive parts with epoxy, acrylic and polyurethane films.
The coatings have excellent scratch resistance and corrosion resistance. Acrylic and polyurethane coatings are also resistant against ultra-violet light.
to top
Related internal links
to Metals
to Coating technologies