to Polymers
Combustion, pyrolysis and gasification of scrap tires
Dr. Dmitri Kopeliovich
Waste tires account about 2% of the global solid waste.
Tires are almost not biodegradable.
More than 1 billion of scrap tires are generated every year in the world. Most of them are dumped into landfills contaminating the environment, providing breeding sites for mosquitoes and presenting a fire risk.
However the environmental aspect is not the only reason to recycle the scrap tires. Dumped tires store a large amount of energy, which may be recovered from them in form of solid, liquid and gaseous fuel.
Combustion
The energy may be recovered from scrap tires directly by using them as a fuel in incinerators.
This technique is called combustion. It allows to recover maximum amount of the energy: 12,000-16,000 BTU/lb (27,900-37,200 kJ/kg). However the direct tire combustion produces black contaminated smoke, which should be cleaned.
to top
Pyrolysis
The alternative approach of scrap tires energy recycling is a decomposition of the tire material by a thermal conversion process.
There are two types of thermal conversion of carbon containing materials: pyrolysis and gasification.
Pyrolysis is the thermal decomposition of an organic substance in the absence of Oxygen (inert atmosphere).
In pyrolysis large hydrocarbon molecules of the substance break down into smaller molecules. Generally three products form as a result of pyrolysis: a fuel gas, liquid (pyrolytic oil)- up to 60% and solid residue (char)- 35-40%.
The solid char is composed of carbon black, which may be used for manufacturing tires and other rubber products, as a pigment in inks, paints and toners, as active carbon and for fabrication electrodes and cell batteries cores.
The liquid fraction consists of a mixture of oils, which may serve as a fuel or a raw material in oil refinery process. The combustion heat of the liquid fraction is 18,000 BTU/lb (42,000 kJ/kg).
The gaseous product is composed of the gases: CO, CO2, H2, CnHm.
The liquid/gas ratio depends on the temperature. The higher the temperature, the lower the liquid/gas ratio.
The typical temperature range of pyrolysis is 750-1800°F (399-982°C).
to top
Gasification
Another type of the thermal decomposition is gasification.
Gasification is the thermal conversion of an organic substance into a combustible gas in the presence of an oxidant (air, steam).
The oxidant (gasification agent) is supplied to the feedstock (e.g. shredered tires) where a series of heterogeneous reactions takes place:
Pyrolysis: Feedstock → C (carbon black) + volatile fraction
C + 1/2O2 → CO
C + O2 → CO2
C + CO2 → 2CO
C + H2O → CO + H2
C + 2H2 → CH4
nC + m/2H2 → CnHm
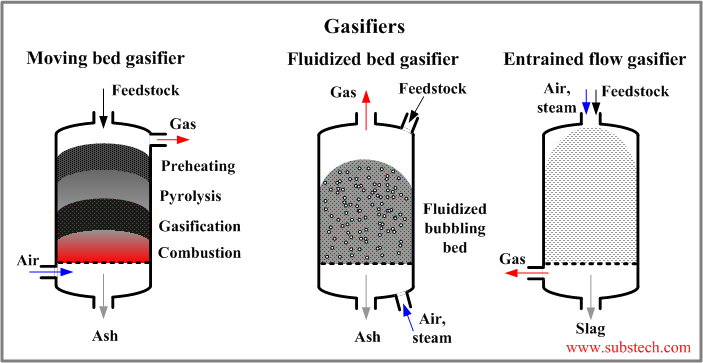
There are three general types of gasification process: fixed bed, fluidized bed and entrained flow (see the picture):
- Fixed (moving) bed gasifier is the simplest type of gasification reactor. The process takes place in a shaft-type reactor. The coarse pieces of tires are loaded from the top. They move downwards. The reactive gas (air, steam, oxygen) comes from the bottom and goes upward in the direction opposite to the direction of the descending feedstock. The feedstock material reacts with the gasification agent producing the product gas, which leaves the gasifier throgh the top. In its way downwards the feedstock passes in series several zones: the drying zone, the pyrolysis zone where the organic substance converts into carbon black, the reductiion (gasification) zone where the oxidizing gases are reduced in the reaction with carbon, the combustion (oxidizing) zone where the residual carbon is oxidized by the oxygen of the gasification agent. The temperature in the combustion zone may reach 2192°F (1200°C). The disadvantages of fixed bed gasifiers are relatively low efficiency and the possibility of the oxidant to break through forming channels in the feedstock and reacting with the gaseous product in form of explosion.
- Fluidized bed gasifier does not have different zones (in contrast to the fixed bed type). The chemical reactions occur in the isothermal dispersion of the fine feedstock particles in the gas. The feedstock particles are mixed with the gase introduced from the bottom of the reactor. The mixture is in a liquid-like form. The gaseous product goes upwards passing through the fluidized bed and leaves the gasifier through the top. Some of the ash particles are taken by the gas and they are separated from it in a cyclone or filters. The temperature of the fluidized bed process is lower than that of the fixed bed: 1292-1652°F (700-900°C).
- Entrained flow gasifier operates with the feedstock particles fed through the top of the reactor. The gasification agent (oxidizing gas) also enters the gasifier through the top. Entrained flow process occurs at high temperature and therefore it takes very short time (few seconds). High temperature also causes the ash to melt forming a slag, which is removed from the gasifier bottom. The gaseous product is clean and should not be additionally cleaned.
Related internal links
to Polymers